How to Define Whether an Electric Vehicle Battery is Suited for Second Life or Not
Author: Mathias Winther Thorsen, ECO STOR
In the last 15 years electric vehicles (EVs) have gone from being something exotic, rare or impractical to becoming a major part of the mass market. As the battery technology moves forward and more producers enter the market, a wide variety of battery packs are put into use. When these batteries reach the end of their life in an EV they could go to recycling, but often they still have significant capacity that can theoretically be put into service in a second life application.
There are, however, some characteristics of the batteries, such as chemistry and design principles, that greatly affect the value and suitability of the batteries in such applications. In this article we will present a few of these key characteristics.
Form Factor
There are three battery cell form factors used in EVs: pouch, prismatic, and cylindrical. These are illustrated in figure 2 below. The pouch and prismatic cells have a higher packing density than cylindrical cells, allowing for high energy density. Pouch cells require a mechanical structure to apply pressure to the cell, while thick cells can be difficult to cool efficiently.
This affects how batteries can be assembled and how thermal management systems are designed for optimal use based on the given application. Variations in the pressure applied to cells depending on their placement will influence their life span, and the same applies for variations in cell temperatures. These variations can even be found within a single cell, giving uneven degradation and a shorter life.
The three different types of battery cells used in EVs contribute to an expanded range of requirements for BMS, BTMS, and fastening techniques, thereby necessitating specialized equipment for disassembly and specialized models and techniques for evaluating battery state of health, safety, reliability, and remaining useful lifetime.
EV batteries are very diverse, and the new anode, cathode, and electrolytes are being researched and developed at a fast pace. Additionally, completely new battery technologies such as all-solid-state batteries, are expected to reach the market in the near future. This makes it challenging to repair, remanufacture, repurpose, and recycle EV batteries, and this technology must follow at the same pace.

Battery Pack Design
Li-ion based EVs are still a relatively new product, where there is rapid development within several different fields and performance indicators. Increased demands on charging speeds, motor power, energy capacity, life span, weight and especially cost of the EV drive the development of battery packs forward, but not necessarily in a direction that is beneficial for battery reuse or repair.
The most common battery pack design principle today is that the pack consists of a number of modules, each consisting of a number of battery cells. If a single battery cell fails, the individual cell can likely not be replaced, but the module can. Similarly, when the battery is reaching the end of its life in an EV, the strongest modules can be reused in a 2nd life application. However, some manufacturers are seeing the module enclosures as unnecessary material and cost as they develop designs where the cells are placed directly into a battery pack, giving a cell-to-pack configuration. This could make both repairs and reuse more difficult, or not feasible at all, as disassembly would damage the battery. A possible solution is reusing the whole battery pack if the weakest cell is still strong enough to work well in a 2nd life application.
Another way to reduce cost, while also improving mechanical properties and waterproofing of the battery pack, is to use glue for assembly of the battery pack. Glue is commonly used to but the outer casing of the battery pack together, meaning that for any repair or reuse of individual modules or cells, the glued surfaces need to come apart. Due to the strength of industrial glue, which is necessary in this application, this operation is very demanding, and can lead to damages to the battery pack. Even if it is generally possible to disassemble the battery pack, this step introduces cost of labour and equipment.
Although some development does not favour 2nd life, there is also some positive development in this regard. New EV battery systems generally have better temperature control systems, which is necessary for higher charging and discharging power levels. This generally gives a better overall operating temperature for the battery cells, and more uniform temperature distribution. This in turn gives longer life span of the battery cells, reduces the chance of degradation of battery safety, and causes the battery cells in the pack to age evenly, enabling reuse of the complete pack after the end of its 1st life.
Different Li-ion Battery Chemistries
Li-ion battery (LIB) chemistries are commonly defined by the material used on the cathode side of the battery. The most common EV cathode materials are NMC (lithium nickel manganese cobalt oxide), LFP (lithium iron phosphate), and NCA (lithium nickel cobalt aluminium oxide). There are variations of the NMC cathode with various fractions of the elements, like NMC111 (1/3 Ni, 1/3 Mn, 1/3 Co), NMC532, NMC622, NMC721, and NMC811, in order of increasing nickel content. The anode material is typically graphite, which has a high capacity for lithium and a high electrical conductivity.
In 2021, the EV cathode market sales shares of nickel-based cathodes (NMC and NCA) were 85%, while the sales shares for LFP were 24% (IEA, 2022). In September 2022, the LFP sales share has increased to 33%, and it is expected to grow (IEA, 2022). NMC has inherently higher specific energy density but suffers from shorter lifetime and lower thermal stability. In addition, fluctuating market prices for nickel and cobalt, and a desire to reduce or remove the use of cobalt, has caused LFP to gain market shares as EV batteries. The next generation batteries, such as various lithium-ion all-solid-state batteries, are expected to reach the market in the near future. For example, Toyota has unveiled its plans to develop all-solid-state batteries for EVs by 2030 (Toyota, 2021).
The cell voltage of the nickel cathodes is between 3.6-4.5 V, while the cell voltage of LFP is between 3.0-3.3V. As a result, the nickel cathodes have a higher energy and power density than LFP cells (J. Jyoti, 2021). On the other hand, LFP has a longer cycle lifetime and is significantly cheaper than nickel-based cathodes. The expected cycling lifetime of LFP is between 2000-3000 cycles, while for nickel cathodes the expected cycling lifetime is between 1000 to 2000 cycles (Battery University, n.d.). The LFP cathodes are considered less prone to entering thermal runaway than nickel-based cathodes, as LFP cells hit this critical point somewhere around 195 C, around 25
C higher than NMC (Duh, et al., 2021).
In addition, the materials in LFP cells are less toxic than substances such as cobalt and nickel in NMC cells (Sun, Bisschop, Niu, & Huang, 2020). NMC cathodes contain higher concentrations of valuable metals (nickel, cobalt, manganese), making them more profitable to recycle than LFP cathodes where the amounts of valuable materials is low (Baum, Bird, Yu, & Ma, 2022). In general, the EV sales share of LFP is expected to increase in the coming years.
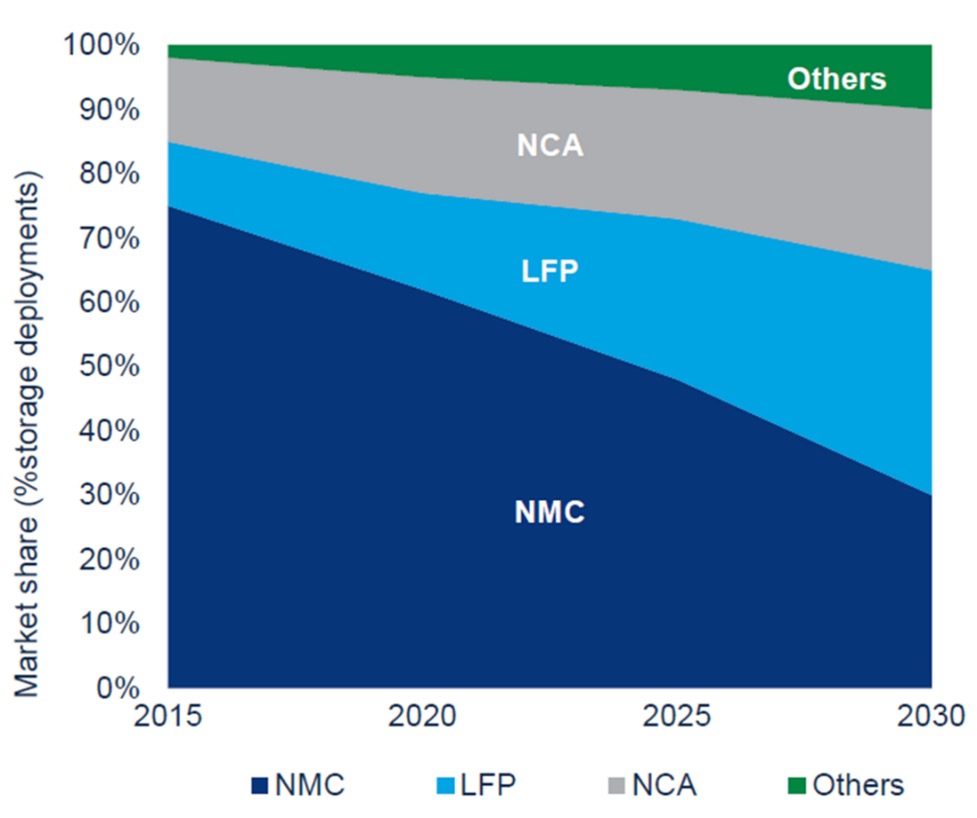
Tesla has used LFP cathode chemistries for some of their models since October 2021. Before that, Tesla has used LiCoO2 (lithium cobalt oxide), NMC, and NCA cathode chemistries for their EVs. BYD also uses LFP cathode chemistries. Audi, Ford, Chevrolet, Hyundai, Jaguar, Nissan, Renault, and VW all use NMC cathodes. Due to the higher stability and longer cycle life of LFP based batteries, these will also be quite suitable for 2nd life use. Another aspect of LFP is that neither iron (Fe) nor phosphorus (P) are particularly valuable materials and current recycling technology is not sufficiently efficient to make it profitable. It is therefore likely that manufacturers will want to get as many cycles from the battery as possible before it’s recycled, e.g. by using it in a 2nd life application.
This has a dual effect, in that the recycling technology is expected to improve significantly until the point where these batteries reach the end of their 2nd life, reducing the cost of recycling. NMC-based batteries on the other hand, contain much more valuable materials which are worth recycling even with current recycling technology. Due to the lower stability of NMC upon extended cycling, it is also likely that fewer of these batteries will be suitable for 2nd life use.

Takeaways
It is clear at this point that all Li-ion electric vehicle (EV) batteries are not the same. There are many parameters that vary between them, giving different characteristics within different areas, resulting in different life span and usability after the battery ends its 1st life.
As the number of EVs worldwide continues to increase rapidly, a large supply of used batteries is expected to emerge. Some batteries will go directly to material recycling where important and scarce minerals can be extracted, while other batteries will be repaired, reused or repurposed for a 2nd life.
Where the different batteries end up is highly dependent on the technology, design and production practices of today.

